Carbon 14 – the post-bomb effect
Friday 9 February 2024
Why is 1954 a carbon-14 frontier? Atomic bombs and atmospheric nuclear testing have artificially increased the amount of carbon-14 in the air. CIRAM laboratories explain the significance of this change.
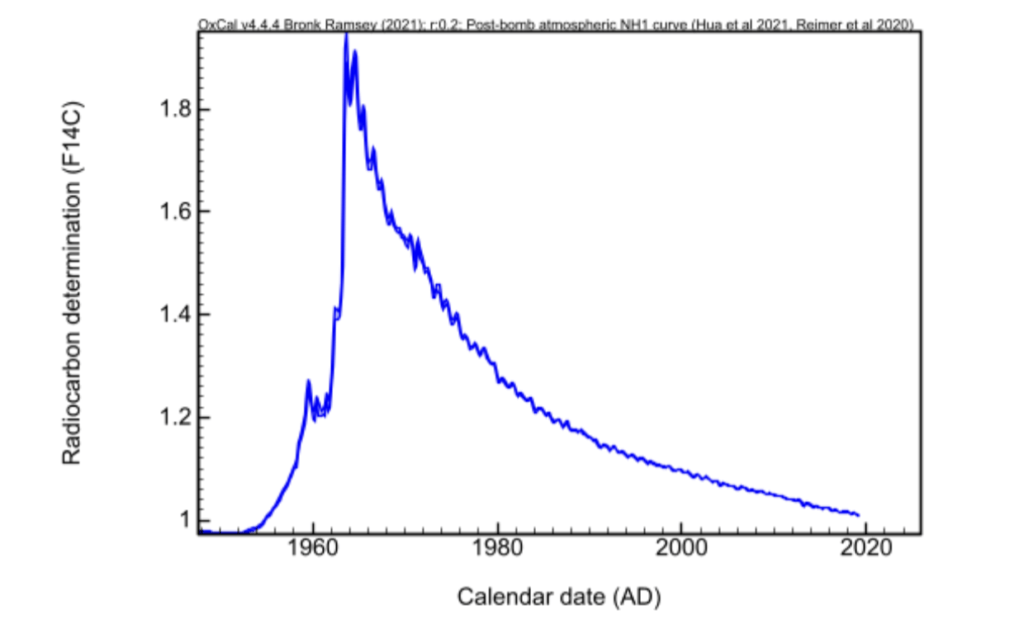
As early as the late 1940s, American researchers began using the properties of natural carbon-14 radioactivity to date organic matter. In the 50s, Nobel Prize-winning chemist Williard Franck Libby successfully dated Egyptian samples.
Today, carbon-14 dating (also known as radiocarbon dating) determines the time elapsed since the death of a living organism. This method has revolutionized archaeometry thanks to its ability to date wood, ivory, bone, teeth, linen, straw and all other organic materials.
